Introduction
The pharmaceutical industry has strict quality requirements for the water used in the production of medicines and other health products. Sterile water, also known as Water for Injection (WFI), is an essential component of these production processes. In this article, we will explore various aspects of WFI, including filtration processes, safety and quality, applications and technology, and regulation and compliance. The use of WFI in pharmaceutical production is crucial to ensuring the integrity and safety of pharmaceutical products. Due to the high standards for water quality and sterile water, WFI plays an indispensable role in the preparation of medicines and other pharmaceutical products.
What is WFI (Water for Injection)?
WFI (water for injection) is sterile water that is used for injection into the human body. Additionally, WFI is utilized in the pharmaceutical industry for the production of pharmaceutical products or for the final rinsing of pharmaceutical packaging. This "purified water" is referred to as sterile water because it contains virtually no foreign substances, such as salts, lime, or microorganisms. Chemically and microbiologically, WFI is sterile enough to meet the quality standards required by the pharmaceutical industry. WFI is essential for pharmaceutical processes and products. Suppliers are authorized to engage third parties for the execution of agreements related to WFI.
How is WFI Produced?
WFI (water for injection) is produced through a combination of distillation and multiple stages of filtration. The filters used for purified water range from coarse pre-filters to sterile membrane filters to remove bacteria. Additionally, some specialized filters may be used, for example, to remove endotoxins or to aerate (storage) tanks. Van Borselen Filters is one of the most experienced suppliers in the Benelux, specializing in filters for the pharmaceutical industry.
Below you will find more information about the possible setups for the production of WFI. At the bottom of the page, there is an overview of all products by process step for this application.
Possible Setups for WFI Production
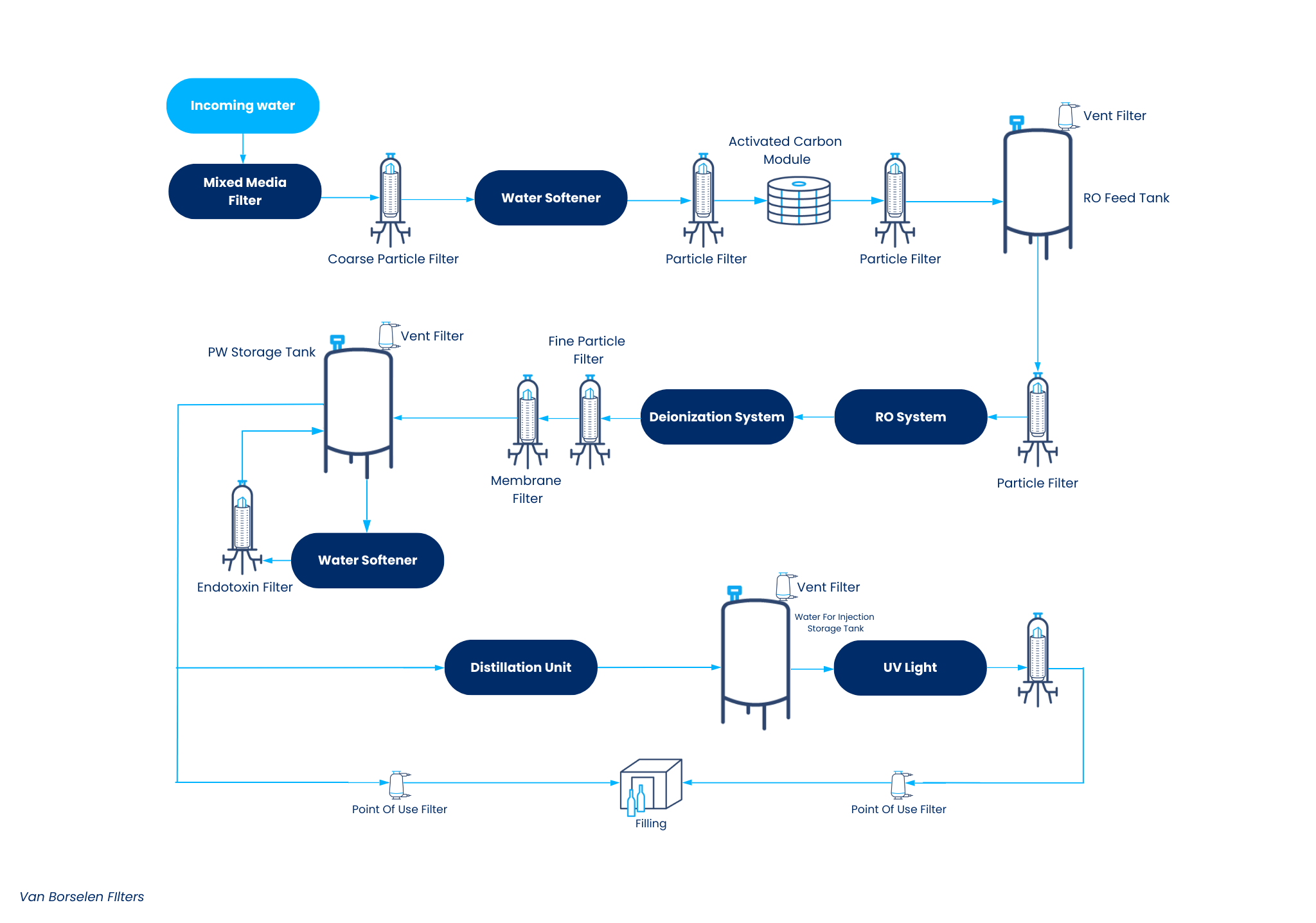
WFI Filtration Processes
The filtration of WFI is a critical process that ensures the water meets the stringent quality requirements of the pharmaceutical industry. There are various filtration processes available, including reverse osmosis, ultrafiltration, and nanofiltration. These processes are designed to remove contaminants and bacteria from the water, ensuring its sterility. Reverse osmosis removes most dissolved solids and impurities by forcing water through a semipermeable membrane. Ultrafiltration uses finer membranes to remove smaller particles and microorganisms. Nanofiltration provides an even higher level of purification by eliminating even the smallest contaminants. The choice of filtration process depends on the specific application and required water quality, with each process making a unique contribution to the production of high-quality WFI.
Which Filters are Used for WFI?
As described above, multiple filters are used in the production of WFI (water for injection). At Van Borselen, we offer filters that meet the highest quality standards to ensure the quality and safety of water for injection. Our sterile filters comply with international regulations, such as USP Class VI, FDA Title 21 (CFR), EC 1935/2004, and GMP. Additionally, the filters and their components are fully traceable and are tested individually for integrity. Naturally, these filters are manufactured in a cleanroom class 8 environment. Finally, our sterile membrane filters come with certificates for quality and traceability.
Safety and Quality
The safety and quality of WFI are of utmost importance in the pharmaceutical industry. The water must meet stringent quality requirements and be free from contaminants and bacteria. The production of WFI must take place in a sterile environment and be executed by experienced personnel. The quality of the water must be continuously monitored and meet the required standards. This includes regular testing and monitoring to ensure that the water consistently meets specifications. Furthermore, all processes and equipment used in the production of WFI must comply with international regulations and standards, such as USP, FDA, and GMP. Through strict controls and high standards, the safety and effectiveness of pharmaceutical products are guaranteed, which is essential for the health and well-being of patients.
Which Product Do You Need for Each Phase?
· Particle filters: geplisseerde filters en capsule filters.
· Vent filters: PTFE filter, PTFE-capsule filter, BorsoCap-SIP, vent filter en P-BE filter.
· Bacteria filter: PES filter en PES-capsule filter, PVDF en nylon
· Point-of-use bacteria filter: PES-capsule filter en PES filter.
Want more about endotoxin filters? Read our case study where you will learn everything about endotoxins and the impact that excessive endotoxins have on the production of medications.


