Common Questions About Filter Integrity Testing
Integrity testing is a requirement for suppliers selling absolute membrane filter cartridges for microbial reduction or the removal of yeast and mold. Test results are validated for the removal of specific organisms, and each filter comes with a certificate. In addition to testing by filter manufacturing companies, users of filter cartridges in various industries perform their own integrity tests for critical processes. In the pharmaceutical industry, regulations require filters to be integrity tested. In the food and beverage industry, although not mandatory, integrity testing is a strategic step to ensure product safety and quality. In this article, we discuss, through a series of questions, the necessity of testing to ensure the integrity of the production process.
Why test the integrity of an already validated filter?
Testing already validated and tested filters is done to ensure that the filter is intact and correctly installed. Once a filter is in production, the only way to determine whether the filter is undamaged and properly installed is by conducting an integrity test. The certificates provided by the manufacturer only indicate that, after production, the filters were intact (meeting the design specifications). Filter performance can be affected by damage during transportation, incorrect installation, chemical cleaning/temperature, and process disruptions.
When should I perform an integrity test?
Since filter integrity testing is used to guarantee that your filters perform as designed, there are many strategies that can be applied. Selecting the appropriate strategy for your process requires evaluating your filtration objectives and product requirements. The more critical your product, the more frequently testing should be performed.
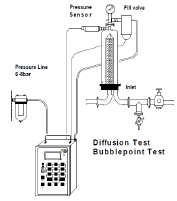
Forward-Flow Diffusion Test
Most filter manufacturers use a Forward-Flow Diffusion Test as a final test before product release. This involves thoroughly wetting the filter (usually with water, but for hydrophobic filters, alcohol/water mixtures can be used), then applying a controlled upstream pressure just below the membrane's bubble point. This allows a diffusion flow through the liquid layer without a bulk flow through open pores. This diffusion flow is validated against bacterial retention to ensure sterilization or bioburden reduction. Filters can be tested manually, but for facilities requiring frequent integrity testing (e.g., before and after every batch), it's advisable to use an automated integrity testing system.
Bubble Point Test
Another option for testing integrity is measuring the bubble point of a filter. This is usually performed on filters before they are installed in the system, but with the appropriate piping, the test can also be conducted after installation. Measuring the bubble point requires installing the filter(s) in a housing and ensuring they are fully wetted with the necessary fluid (typically water for hydrophilic filters or an alcohol/water mixture for hydrophobic filters). The housing is connected to an upstream air source, and the downstream line is directed into a vessel with water where bubbles can be observed. The upstream pressure is gradually increased until a consistent stream of air bubbles is observed in the downstream vessel. If the bubbles form according to the filter's specifications, this indicates that the filter is undamaged and suitable for use.
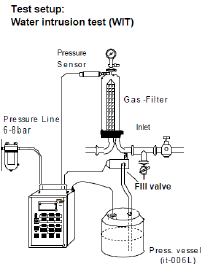
Water Intrusion Test
For testing hydrophobic filters, a water intrusion test can be performed. The water intrusion test is used when alcohol is not permitted in the process. This involves installing the filter(s) in a housing and applying water pressure on the upstream side at a pressure specified by the manufacturer, while measuring any downstream water flow. A hydrophobic filter will repel water due to its hydrophobic properties and thus allow very little water to pass through. These values are specified by the manufacturer. If the water flow downstream exceeds the manufacturer’s specifications, it indicates possible damage or improper installation.
Do you need Support with your in-process integrity testing?
Process integrity testing is crucial for ensuring the quality of your final product by verifying that the filters operate as needed throughout the entire process. This reduces the risk and costs associated with discarding a poor-quality batch.
We are here to assist you. An evaluation is as simple as a conference call with our account managers to review your current situation and provide recommendations on how and where to implement integrity testing for your process, or to supply the appropriate integrity tester. For more information about incorporating integrity testing into your process, please contact us at: +31 (0)79-3412314 or info@vanborselen.nl

